MENU
RADI COVID-19 Ag Rapid test is a rapid immuno-chromatographic assay for qualitative detection of specific antigens to SARS-CoV-2-IgG & IgM present in human nasopharynx. This test contains colloid gold conjugate pad and a membrane strip pre-coated with antibodies specific to SARS-CoV-2 antigen on the test lines.
| RADI COVID-19 Ag Rapid Test | |
|---|---|
| Intended use | A lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal swab specimens. |
| Specimen type | Nasopharyngeal Swab |
| Test / kit | 25 tests / kit |
| Storage Condition | 2~30 ℃ |
| Shelf life | 24 months |
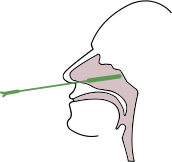
Insert a sterile swab into the nostril of the patient, and swab over the surface of the posterior nasopharynx. Withdraw the swab from the nasal cavity.
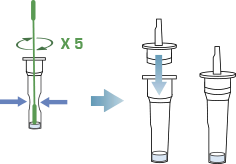
Insert the swab into an extraction tube. While squeezing the buffer tube, stir the swab more than 5 times strongly. Remove the swab while squeezing the sides of the tube to extract the liquid from the swab and screw the filter cap tightly onto the tube.
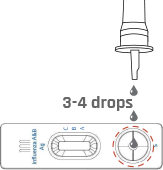
Apply 3 drops of extracted specimen to the specimen well of the test device.

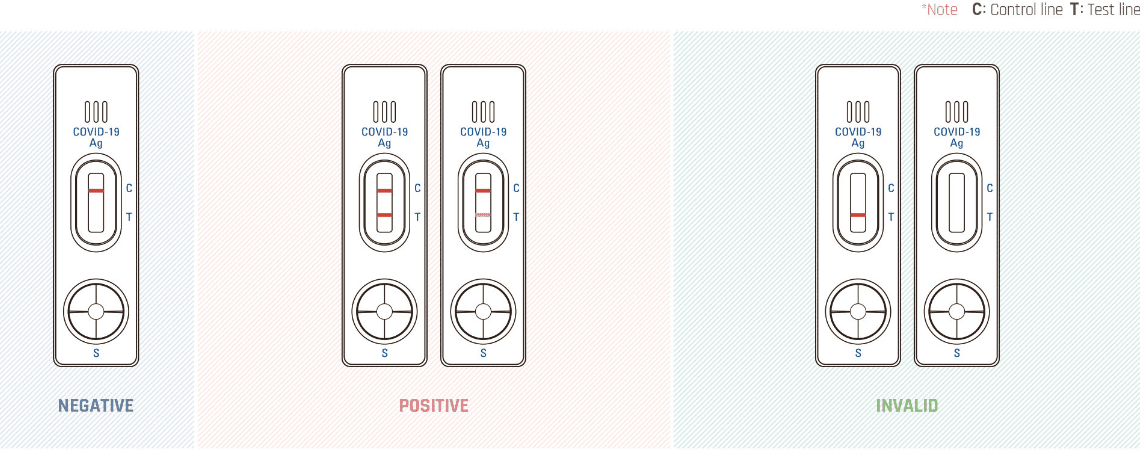
Read the test result at 15 minutes.
1) Clinical evaluation in USA
The clinical performance validation of RADI COVID-19 Ag Rapid Test was evaluated in the high complexity laboratory certified to conduct both molecular and serological diagnosis in the United States(U.S.) with 111 de-identified positive and 120 deidentified
negative nasopharyngeal swab sample in VTM, which were confirmed by
DTPM COVID- 19 RT PCR test with an Emergency Use Authorization (EUA) granted by the FDA (Comparator method).
The leftover de-identified samples used had Ct values ranging from 15.081 to 33.696 and the cut-off values for the EUA authorized DTPM molecular RT-qPCR assay is 35 cycles.
As a result, the RADI COVID-19 Ag Rapid Test had 100% specificity and 97.3% sensitivity for nasopharyngeal samples when using Ct-values less than 35 cycles as a cut-off for comparator method (DTPM COVID- 19 RT PCR test).
| CLINICAL PERFORMANCE | DTPM COVID- 19 RT PCR TEST (Comparator Method) |
|||
| Positive | Negative | Total | ||
| RADI COVID-19 Ag Rapid Test | Positive | 108 | 0 | 108 |
|---|---|---|---|---|
| Negative | 3 | 120 | 123 | |
| Total | 111 | 120 | 231 | |
| Sensitivity | 97.30% (95 CI (%): 92.4 to 99.1%) | |||
| Specificity | 100% (95 CI (%): 96.9 to 100%) | |||
| Total Agreement | 98.70% | |||
2) Clinical evaluation in Ghana
The RADI COVID-19 Ag Rapid Test Kit was evaluated on 8th February 2021 by NMIMR(Noguchi Memorial Institute For Medical Research), University of Ghana.
Leftover nasopharyngeal clinical specimens after routine diagnosis were enrolled. (Specimen Collection Period : ( January -February 2021). A total of 94 nasopharyngeal clinical specimens in VTM were randomly selected with variation in their Ct values which is consist of 39 SARS Cov-2 positive and 55 SARS-CoV-2 negative confirmed by Q-PCR(Detection kit for 2019-nCoV RNA (PCR Fluorescence Probing)) as comparator method which is a product included in the World Health Organization Emergency Use List(EUL).
The cut-off values for the Q-PCR assay is 40 cycles. The range of Ct value regarding positive samples was 15.41 to 23.62 (ORF1ab gene) and 14.2 to 25.96 (N gene). The Evaluation of RADI COVID-19 Ag Rapid Test was conducted in blinded fashion. The performance of RADI COVID-19 Ag Rapid test was shown in comparison with Comparator method as below.
| CLINICAL PERFORMANCE | Detection kit for 2019-nCoV RNA (PCR Fluorescence Probing) (WHO EUL test) |
|||
| Positive | Negative | Total | ||
| RADI COVID-19 Ag Rapid Test | Positive | 37 | 3 | 40 |
|---|---|---|---|---|
| Negative | 2 | 52 | 54 | |
| Total | 39 | 55 | 94 | |
| Sensitivity | 94.9% (95%CI : 83.1 to 98.6) | |||
| Specificity | 94.6% (95%CI : 85.1 to 98.1) | |||
3) Clinical evaluation in Cameroon
RADI COVID-19 Ag Rapid Test kit was evaluated between February 1st and March 31st, 2021 by Epicentre (the research arm of Médecins Sans Frontières) in Yaoundé, Cameroon using fresh samples collected from symptomatic patients indiscriminately with respect to time from symptom onset.
A total of 127 fresh nasopharyngeal specimens with varying Ct values consisting of 33 SARS-CoV-2 positive and 94 SARS-CoV-2 negatives confirmed by RT-PCR(Abbott RT-PCR) were used. Collection of all samples was done less than 5 days from symptom onset.
The performance of RADI COVID-19 Ag Rapid test was shown in comparison with Comparator method as below.
| CLINICAL PERFORMANCE | Abbott RT-PCR (USFDA EUA test) |
|||
| Positive | Negative | Total | ||
| RADI COVID-19 Ag Rapid Test | Positive | 28 | 2 | 30 |
|---|---|---|---|---|
| Negative | 5 | 92 | 97 | |
| Total | 33 | 94 | 127 | |
| Sensitivity | 84.8% (95% CI 69.1 to 93.3%) | |||
| Specificity | 97.9% (95% CI 92.6 to 99.4%) | |||
The RADI COVID-19 Ag Rapid Test has established the limit of detection by two different methods (VTM method / direct swab method) with serial diluted concentration of heat inactivated SARS-CoV-2 Isolate in negative nasopharyngeal clinical matrix. (Source of Material used: Hong Kong/VM20001061/2020, concentration : 1.15 x 107 TCID50/mL, Manufacturer : Zeptometrix).
1) VTM method: the heat inactivated virus was diluted with negative clinical matrix in VTM to make different viral diluted concentration (initial test concentration : 1.15 x 107 TCID50/mL).
2) Direct swab method: Spiking 30 ul of the heat inactivated virus to a swab and dilution with negative clinical matrix (Initial test concentration : 9.86 x105 TCID50/mL)
Test for each method was done according to the instructions for use.
Once tentative confirmed LoD was established from different viral diluted concentrations for both VTM and direct swab method, the final LoD of each method was determined at the lowest virus concentration level where the product can be detectable more than 95%(19/20) detection rate in 20 replicates.
| METHOD | LoD |
| VTM METHOD | 1.43 x103 TCID50/mL |
| DIRECT SWAB METHOD | 9.86 x101 TCID50/mL (≒2.96 x100 TCID50/Swab) |
Totally, 35 kinds of microorganisms following FDA EUA guideline were evaluated in the study by wet-testing of 30 microorganisms and in-silico analysis of 5 microorganisms. No cross-reactivity or interference was seen in the study.
Wet testing
| NO. | PATHOGEN |
| 1 | Coronavirus (Strain: 229E) |
| 2 | Coronavirus (Strain: OC43) |
| 3 | Coronavirus (Strain: NL63) |
| 4 | Coronavirus MERS |
| 5 | Adenovirus |
| 6 | Influenza A (H1N1) |
| 7 | Influenza A (H3N2) |
| 8 | Influenza B |
| 9 | Human Metapneumovirus (hMPV) |
| 10 | Parainfluenza Virus Type 1 |
| 11 | Parainfluenza Virus Type 2 |
| 12 | Parainfluenza Virus Type 3 |
| 13 | Parainfluenza Virus Type 4 |
| 14 | Rhinovirus |
| 15 | Respiratory syncytial virus (Type A) |
| 16 | Respiratory syncytial virus (Type B) |
| NO. | PATHOGEN |
| 17 | SARS-coronavirus |
| 18 | Enterovirus A |
| 19 | Enterovirus B |
| 20 | Enterovirus C |
| 21 | Enterovirus D |
| 22 | Haemophilus influenzae |
| 23 | Streptococcus pneumoniae |
| 24 | Streptococcus pyogenes |
| 25 | Candida albicans |
| 26 | Bordetella pertussis |
| 27 | Legionella pneumophila |
| 28 | Staphylococcus aureus |
| 29 | Staphylococcus epidermidis |
| 30 | Pooled human nasal wash – representative of normal respiratory microbial flora |
In-silico analysis
| NO. | PATHOGEN |
| 31 | Mycoplasma pneumoniae 1 |
| 32 | Chlamydia pneumoniae 1 |
| 33 | Mycobacterium tuberculosis 1 |
| NO. | PATHOGEN |
| 34 | Pneumocystis jirovecii (PJP) 1 |
| 35 | Human coronavirus HKU1 2 |
1No significant similarity found. Homology-based cross-reactivity can be ruled out.
2The comparison analysis revealed a 23% homology across 82% of the sequences. the likelihood of cross reactivity of human coronavirus HKU1 cannot be ruled out.
RADI COVID-19 Ag Rapid Test has been tested with 20 potentially interfering endogenous or exogenous substances in negative clinical matrix with the absence or presence of SARS-CoV-2 (3X LoD). No inference was seen in the study.
| NO. | SUBSTANCE | ACTIVE INGREDIENT | CONCENTRATION |
| 1 | Endogenous | Mucin | 0.5 % |
| 2 | Endogenous | Whole blood | 4 % |
| 3 | Nasal spray | Cromolyn | 15 % |
| 4 | Nasal drops | Phenylephrine | 15 % |
| 5 | Nasal gel | Sodium Chloride | 5 % |
| 6 | Respiratory Specimens | Biotin | 100 ug/ml |
| 7 | Antibiotic | Chloramphenicol | 100 mg/dL |
| 8 | Anti-inflammatory medication | Acetaminophen | 10 mg/ml |
| 9 | Throat lozenges | Benzocaine | 1.5 mg/ml |
| 10 | Anti-viral drugs | Oseltamivir Phosphate | 5 mg/ml |
| 11 | Antibiotic, Nasal Ointment | Mupirocin | 10 mg/ml |
| 12 | Antibacterial, Systemic | Tobramycin | 4 ug/ml |
| 13 | Therapeutics/Drug | Dexamethasone | 1 mg/ml |
| 14 | Endogenous | Oxymetazoline | 15 % |
| 15 | Endogenous | Fluticasone Propionate | 5 % |
| 16 | Endogenous | Menthol | 1.5 mg/ml |
| 17 | Naso GEL (NeilMed)* | Sodium bicarbonate | 5% |
| 18 | Zicam* | zinc acetate | 5 % |
| 19 | Homeopathic (Alkalol)* | Eucalyptol | 1:10 dilution |
| 20 | Sore Throat Phenol Spray* | Phenol | 15 %(v/v) |
* : For the list of substances highlighted with “*”, due to difficulty to secure branded commercial products, we tested its active ingredients for likelihood of potential interference in the consideration of main active ingredient of the concerning products.