MENU
The RADI Human Papillomavirus Detection KIT is an in vitro diagnostic medical device,
based on Real-time RT-PCR technology to screen the HPV DNA.
| RADI Human Papillomavirus Detection KIT (REF: RS002) | |
|---|---|
| Intended use | Qualitative screening of nucleic acid extracted from the 2 High risk HPV 16 (HEX) and HPV18 (ROX) and the other comparative low risk genotypes (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68-FAM) |
| Specimen type | Urine, Vaginal Swab |
| Target | HPV16 and/0r HPV18 and/or HPC others |
| Product contents |
HPV Primer & Probe mixture 2X RADI MasterMix HPV Positive control RNase free water |
| LoD (copies/㎕) |
Urine: : 575.44 copies (HPV16), 446.68 copies (HPV18), 371.19~1000 copies (HPV others) Vaginal Swab: 645.65 copies (HPV16), 588.84 copies (HPV18), 331.13~1230.27 copies (HPV others) |
| Storage condition |
-25 ℃ ~ -15 ℃ |
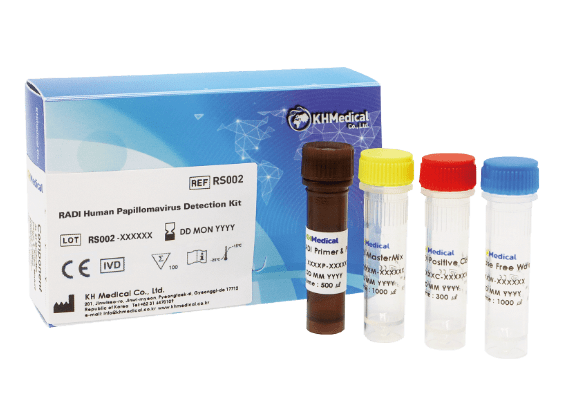


* Simplified Procedure when make mixture
(Number of tubes minimized)!