MENU
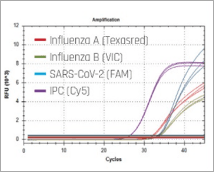
The RADI FAST Influenza A/B Detection Kit is an in vitro diagnostic medical device,
based on Real-time RT-PCR technology utilizing reverse-transcriptase (RT) reaction to convert RNA into complementary DNA (cDNA).
| RADI FAST Influenza A/B Detection Kit (REF: RV011F) | |
|---|---|
| Intended use | Qualitative detection of nucleic acid extracted from the Influenza virus in Upper respiratory specimens such as Nasopharyngeal swab. This kit is intended for the differential detection of the Influenza genotypes A and B. |
| Specimen type | Respiratory specimens such as nasopharyngeal swabs specimens from symptomatic individuals suspected of Influenza A/B. |
| Target | Influenza A/B |
| Product contents |
Influenza A/B Primer & Probe mixture 2X RADI FAST MasterMix Influenza A/B Positive control RNase free water |
| LoD (copies/㎕) | 4.9 copies |
| Storage condition |
-25 ℃ ~ -15 ℃ |
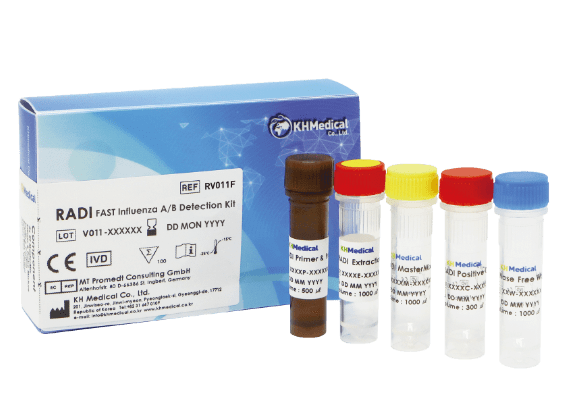


* Simplified Procedure when make mixture
(Number of tubes minimized)!